Professor Terri Camesano has a paper accepted for publication by ACS Applied Materials & Interfaces.
Creating Antibacterial Surfaces with the Peptide Chrysophsin-1
Abstract
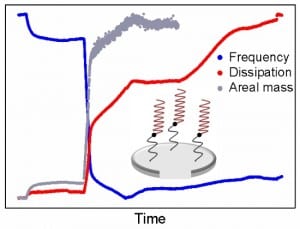
Immobilization of antimicrobial peptides (AMPs) holds potential for creating surfaces with bactericidal properties. In order to successfully incorporate AMPs into desired materials, increased fundamental understanding of the relationship between AMP immobilization and the efficacy of bound peptides as antibacterial agents is required. In this study, we characterize the relationship between surface binding of the AMP and subsequent ability of the peptide to kill bacteria. Surface immobilization of the AMP chrysophsin-1 (CHY1) via a flexible linker is studied in real-time, using a quartz crystal microbalance with dissipation monitoring (QCM-D). Depending on whether the AMP is physically adsorbed to the surface or attached covalently via a zero-length or flexible cross-linker, changes could be observed in AMP orientation, surface density, flexibility, and activity against bacteria. Covalent surface binding of CHY1 led to the formation of solvated monolayers of vertically positioned peptide molecules, while the physical adsorption of CHY1 led to the deposition of rigid monolayers of horizontally positioned peptide molecules on the sensor surface. Covalently bound peptides were not removed by extensive washing and did not leach from the surface. Zero-length immobilization of the peptide decreased its ability to kill E. coli to 34% ± 7% of added bacteria, while binding via a flexible linker resulted in 82% ± 11% of bacteria being killed by the AMP.